I smiled when I saw the perchloric acid part.
We've more or less seen molecular models of clubs in chemistry class, and you may have spelled them yourself. When these molecular models were born, they didn't look the same as they do now.
the first chemist to use molecular models in public speaking was Auguste William von Hoffman (August Wilhelm von Hofmann), who made many important contributions in the field of organic chemistry, and chemistry students will learn several reactions named after him. In the 1860s, Hoffman began to represent molecular structures with colored balls and sticks, and the model atomic color matching at that time is still used today: black for carbon, white for hydrogen, red for oxygen, and blue for nitrogen.
here is a physical model of Hoffman's molecular model of methane:
(collected by the Royal Institute, which Hoffman used in his speech in 1865)
obviously, this is very different from the regular tetrahedron we see in textbooks. At that time, chemists had begun to understand the composition of molecules, but they knew very little about the specific spatial structure of molecules (the tetravalent nature of carbon was still very new in the 1860s). As a result, Hoffman made the methane molecular model into a horizontal and vertical plane structure according to his own understanding.
other molecular models he designed are in a similar style. For example, straight-line water molecules:
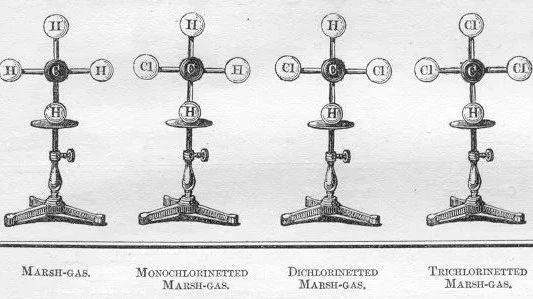
all kinds of alkanes and chloroalkanes:
of course, I think the most exciting is the following. Chloric acid molecules of various oxidized states:
from left to right are hydrochloric acid, hypochlorite, chlorite, chloric acid and perchloric acid. This idea can be said to be very simple and easy to understand. (no) _ (: "∠") _
Photo Source: https://webspace.yale.edu/chem125/125/history99/6Stereochemistry/models/models.html
the first chemist to use molecular models in public speaking was Auguste William von Hoffman (August Wilhelm von Hofmann), who made many important contributions in the field of organic chemistry, and chemistry students will learn several reactions named after him. In the 1860s, Hoffman began to represent molecular structures with colored balls and sticks, and the model atomic color matching at that time is still used today: black for carbon, white for hydrogen, red for oxygen, and blue for nitrogen.
here is a physical model of Hoffman's molecular model of methane:
(collected by the Royal Institute, which Hoffman used in his speech in 1865)
obviously, this is very different from the regular tetrahedron we see in textbooks. At that time, chemists had begun to understand the composition of molecules, but they knew very little about the specific spatial structure of molecules (the tetravalent nature of carbon was still very new in the 1860s). As a result, Hoffman made the methane molecular model into a horizontal and vertical plane structure according to his own understanding.
other molecular models he designed are in a similar style. For example, straight-line water molecules:
all kinds of alkanes and chloroalkanes:
of course, I think the most exciting is the following. Chloric acid molecules of various oxidized states:
from left to right are hydrochloric acid, hypochlorite, chlorite, chloric acid and perchloric acid. This idea can be said to be very simple and easy to understand. (no) _ (: "∠") _
Our wedding dresses for older brides 2nd marriage are a perfect combination of comfort and elegance and fit any occasion. Our trendy collections are surprisingly affordable.
Photo Source: https://webspace.yale.edu/chem125/125/history99/6Stereochemistry/models/models.html